
Iso 14971 how to#
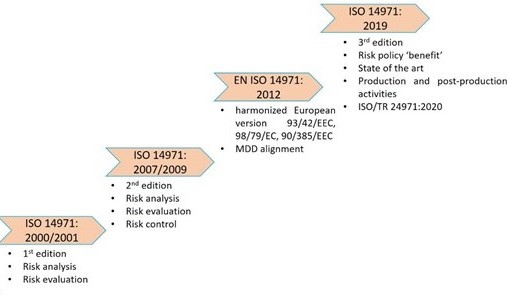
“This guidance has gone beyond the previous version of the TIR and the standard to answer all the questions that organizations had about how to implement life-cycle risk management within their organization,” said Tina Krenc, principal consultant for KTA Compliance Consulting, lead instructor for AAMI’s industry training course on Integrating Risk Management into the Product Life Cycle, and a member of the joint working group. “The revision of both the risk management standard and the TIR reflect changes in regulatory requirements, not only the Medical Device Regulations (MDR) in the European Union, but also with more focus on benefits and risks in several other jurisdictions.”

“The revision intends to bring the standard fully in line with regulatory expectations, so that it is fit for the next decade,” said Jos van Vroonhoven, senior manager of standardization for Global Regulation & Standards at Philips and convener of ISO/TC 210 – IEC/SC 62A JWG1. Users of the risk management standard now have a more complete and updated view of how to implement it in their organization so that our device users, patients, and stakeholders have safer medical devices.” “The informative annexes were nearly completely rewritten. “The resulting document has 38 new pages of informative annexes over the previous versions of these two documents, for a total of 89 pages of guidance,” Bills said.
Iso 14971 iso#
TIR24971 is the result of three years of intensive effort by the joint working group to align the guidance with the revised ISO 14971 standard, according to Edwin Bills, a consultant and a member of the group. “The revised guidance helps to ensure all are understanding and applying the principles of risk management in a harmonized, repeatable way, which will ultimately improve the safety and efficacy of medical devices.” “Risk management as a topic is only becoming more important to the medical device industry as devices are becoming more technically complex and manufactures push innovation,” said Wil Vargas, senior director of standards at AAMI. “It is a must-have for anyone in the medical device industry to fully understand the concepts and intent as they look to apply risk management to meet ISO 14971 and the applicable regulatory requirements.The standard and TIR were developed by ISO TC210 – Quality management and corresponding general aspects for medical devices, JWG1 – Application of risk management to medical devices. “TIR24971 is the document that industry has been waiting for to apply risk management,” said Mark Swanson, president of H&M Consulting Group, LLC, and a member of the joint working group. It is a companion piece intended to be used and applied together with the standard, ANSI/AAMI/ISO 14971:2019, Medical devices-Application of risk management to medical devices, which establishes a process for medical device manufacturers to identify, evaluate, and manage risk. The TIR offers guidance on management responsibilities, components of a risk management plan, and the risk analysis and evaluation process. Media contact: Brian Stallard ( long-awaited technical information report (TIR) that provides state-of-the-art guidance on applying a fundamental risk management standard has just been published.Īlready, AAMI/ISO TIR24971:2020, Medical devices-Guidance on the application of ISO 14971, has been a hot seller as a draft document.

Risk management can be an integral part of a quality management system. This document requires manufacturers to establish objective criteria for risk acceptability but does not specify acceptable risk levels. decisions on the use of a medical device in the context of any particular clinical procedure or The process described in this document can also be applied to products that are not necessarily medical devices in some jurisdictions and can also be used by others involved in the medical device life cycle. The process described in this document applies to risks associated with a medical device, such as risks related to biocompatibility, data and systems security, electricity, moving parts, radiation, and usability. The requirements of this document are applicable to all phases of the life cycle of a medical device.
The process described in this document intends to assist manufacturers of medical devices to identify the hazards associated with the medical device, to estimate and evaluate the associated risks, to control these risks, and to monitor the effectiveness of the controls.
Iso 14971 software#
This document specifies terminology, principles and a process for risk management of medical devices, including software as a medical device and in vitro diagnostic medical devices.


 0 kommentar(er)
0 kommentar(er)
